Thermodynamic cycles
A thermodynamic cycle is a linked series of processes such that the outlet of the final process is the same state as the input of the first process. It will be seen later that when a thermodynamic cycle is used to convert heat into work, heat must necessarily be rejected from the cycle. Figure 1 shows the block diagram of a generalised cycle. At various points heat and work are input and output. Two important quantities are the thermal efficiency of the cycle, ηth, and the work ratio, rw defined by:
The thermal efficiency is a measure of how much of the input heat is converted into useful work, and makes the assumption that the heat rejected in the cycle is valueless. This is often, but not always, true. In an internal combustion engine the input heat is the heat liberated by the burning of the fuel. Of course a good cycle has a high thermal efficiency. A consequence of the first law of thermodynamics is that the maximum value possible for the thermal efficiency is 1.
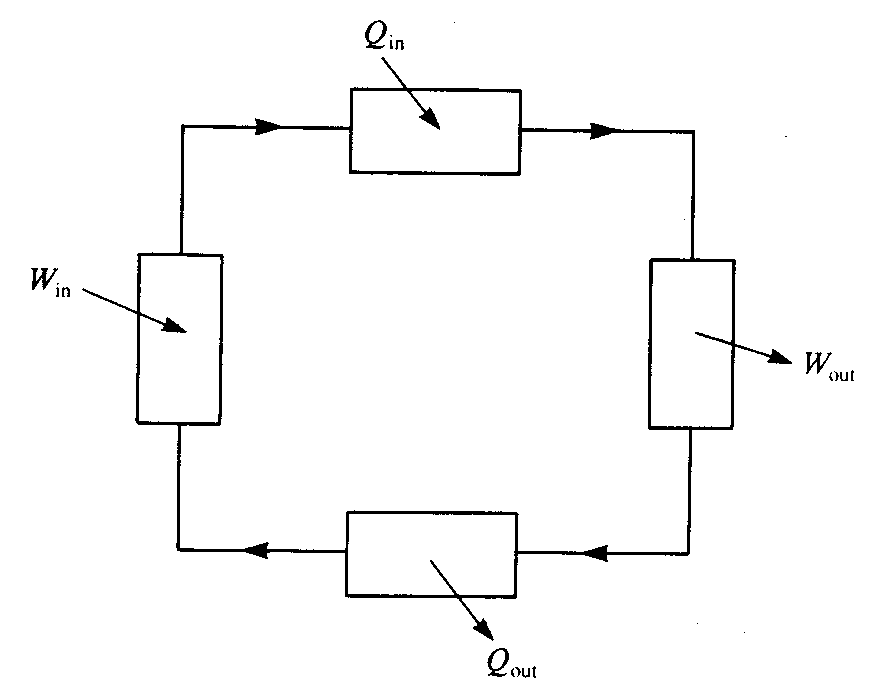
Figure 1 A block diagram of a generalised thermodynamic cycle showing
transfers of heat and work in and out of the cycle}
The work ratio measures the relative sizes of the work out and the work in terms in Figure 1. If they are of comparable size, giving a low work ratio, then there are two undesirable consequences:
For a given value of the net work out, individual terms Wout and Win will be large. Thus large machines will be required to handle these large work transfers.
If the machines forming the cycle are not as mechanically efficient as expected, then Wout will be smaller than expected, and Win will be smaller than expected. This has the effect of drastically reducing the net work output below its expected value.
Hence a high work ratio is a desirable aim. The maximum value of the work ratio is 1.
from Basic Engineering ThermodynamicsBasic_Engineering_Thermodynamics, by Peter B. Whalley. Reprinted with permission of Oxford University Press
Created with the Personal Edition of HelpNDoc: Generate Kindle eBooks with ease