Thermodynamic systems and properties
In thermodynamics the totality of the world is usually termed the universe, and is divided into two parts.
1. The system is the part of the universe in which we are particularly interested.
2. The rest of the universe is the surroundings.
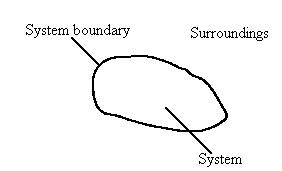
Figure 1 The system, the surroundings, and the system boundary
Separating the system from the surroundings is the system boundary, see Figure 1. Systems can be of three types depending on what can pass through the system boundary.
1. If matter, heat and work can pass through the system boundary, then the system is an open system.
2. If heat and work can pass through the boundary, but matter cannot, then the system is a closed system.
3. If neither matter, not heat, nor work can pass through the boundary, then the system is an isolated system.
The various types of system are summarised in Table 1.
Table 1 The various types of system
Boundary permeable to:
System type matter heat work
isolated no no no
closed no yes yes
open yes yes yes
The system might typically be a certain mass of gas, or a mixture of liquid and gas (such as water and steam). The condition of the system is identified by a number of properties of state such as the temperature, pressure, and volume. It will be noted later that heat and work are not properties of state like these other properties.
The phase rule specifies how many properties of state are necessary to define the system, see Table 2. It is often stated that two properties are necessary to specify a system, and this will be used frequently later, but it must be remembered that this is only true for a single phase system.
Table 2 Number of properties of state required to specify a system with a single pure component
Number of properties
Number of Phases of state required
1 2
2 1
3 0
Properties are sometimes divided into two types:
1. Extensive properties which depend on the mass of substance present, for example volume. The greater the mass of air, the greater will be the volume.
2. Intensive properties which do not depend on the mass of substance present, for example pressure and temperature. Specific volume is defined as the volume per unit mass, and so must be an intensive property.
Extensive properties are usually given an upper case symbol, for example V for volume. Intensive properties are usually given a lower case symbol, so p for pressure, and v for specific volume. However the use of T for the intensive property temperature is an exception to this rule.
from Basic Engineering ThermodynamicsBasic_Engineering_Thermodynamics, by Peter B. Whalley. Reprinted with permission of Oxford University Press
Created with the Personal Edition of HelpNDoc: Easily create PDF Help documents